Abstract
Background Lymphocyte predominant (LP) cells located outside B-cell-rich nodules are the hallmark of variant morphologic patterns of nodular lymphocyte predominant Hodgkin lymphoma (NLPHL). In comparison with typical NLPHL (patterns A and B), cases with variant morpholoy (patterns C, D, E and F) are associated with significantly worse clinical behavior. Nevertheless, assessment of tissue architecture may be challenging in limited biopsy specimens, which are increasingly common in routine clinical practice. We sought to address this gap by developing artificial intelligence-assisted modules to accurately recognize NLPHL and distinguish patterns A/B from variant patterns, namely the most common of those, pattern C.
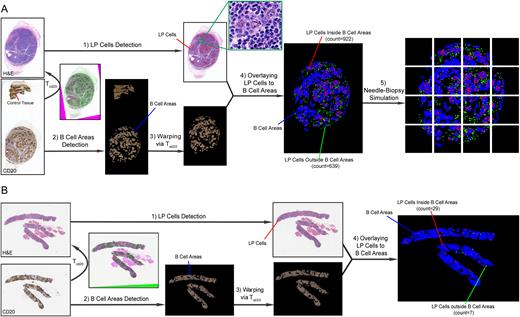
Material & Methods The study group included whole slide images (WSI) of hematoxylin and eosin (H&E) stains and CD20 immunohistochemistry stains from 10 NLPHL patients; cases emcompassed patterns A, B & C (A/B: 5 cases; C: 5 cases) as determined by hematopathology review. We designed modules to assess the spatial relationship between LP cells and B cell-rich areas by first seeking to detect LP cells using high-power field (HPF) magnification of H&E WSI (Figure A1). We split the WSI into tiles (8000 x 8000 pixels) to mitigate the computational burden of WSI analysis. For each tile, we deployed the pretrained HoVer-Net for nuclear segmentation. To distinguish LP cells from surrounding small lymphocytes, we analyzed nuclear size, intensity, and gray-level co-courrance matrix (GLCM) features (correlation and dissimilarity). For each feature, we identified the optimal threshold based on feature histogram of LP in comparison to the remainder surrounding cells. Nuclei meeting all feature criteria were labeled as LP cells and validated morphologically by hematopathologists. All tiles were then stitched to illustrate the spatial distribution of LP cells on WSI. Next we deployed the active contour (AC) technique, an iterative region-growing image segmentation algorithm, to identify B-cell areas from CD20 stains (Figure A2). To reliably define the intial contours for the AC, we abided by the following criteria: 1) Red (R) channel intensity larger than both the green (G) and blue (B) channels, with a ratio of 1.25; 2) Including pixels in the bottom 10% of the intensity histogram after removal of slide background and converting the RGB image into gray scale. We merged the contours from these two techniques and used them as initial seeds for AC segmentation, after which we took the final evolved contour as the boundaries of B-cell areas. We then overlayed CD20 on H&E WSI to assess the spatial relationship between the detected LP cells and B-cell areas. Since tissue sections on the CD20 and H&E slides represent neighboring slices, only translation, rotation, and slight scaling were surmised to occurr between slides in this pilot study set. Accordingly, we employed the affine registration paradigm and optimized mutual information between the transformed CD20 and fixed H&E images until convergence then warped the detected B-cell areas via the obtained deformation field (Figure A3).
Results The concordant positioning between two stains after registration allowed us to compare the spatial relationship of LP cells to B-cell areas (including assessment of the number of LP cells inside (922) and outside (639) B-cell areas) (Figure A4). We then segmented the final image into quadrants to simulate needle core biopsies and confirmed the efficiency of our modules in detecting LP cells and analyzing their relationship to B-cell areas in limited tissue, in all quadrants (Figure A5). Our model was successfully applied on 100% of 10 NLPHL cases in this pilot group. After proof of concept was established in a full tissue biopsy sample (Figure A1-5), we applied the same steps to an actual physical needle core biopsy of an NLPHL-pattern C case (Figure B1-3) and were able to identify 7 out of 29 LP cells located outside B-cell areas, in congruence with the previously rendered diagnosis (Figure B4).
Conclusion This technique, using H&E staining and CD20 immunohistochemistry only, is especially promising to work-up cases when ordering a routine panel of immunohistochemical stains is not feasible due to limited tissue that may be exhausted. Further validation using an independent cohort is warranted in the future.
Disclosures
Loghavi:Amgen: Research Funding; Astellas: Research Funding; QualWorld: Consultancy; Abbvie: Consultancy, Current equity holder in publicly-traded company; GLG: Consultancy; PeerView: Honoraria. Reagan:Genentech: Research Funding; Seagen: Research Funding; Kite, a Gilead Company: Consultancy; Caribou Biosciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal